Growth Factor Identification Based on Speed and Duration of Centrifugation in Platelet Rich Plasma
Main Article Content
Abstract
Background: This study aims to determine the appropriate preparation of simple PRP protocol to yield the maximum growth factor concentration, specifically for PDGF and TGF-β1.
Methods: Blood samples were collected from 5 healthy volunteers who signed informed consent for participation in the study. The samples then processed by single centrifugation at 4 four different speed (600, 800, 1000, and 1200 rpm) for 4 different centrifugation times ( 8, 10, 12, 14 minutes). The Platelet Derived Growth Factor (PDGF) and Transforming Growth Factor β1 (TGF- β1) concentrations were determined by enzyme-linked immunosorbent assay (ELISA). Blood routine test analysis were measured by hematology analyzer.
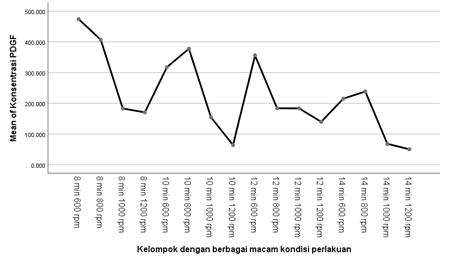
Results: The 8 min with 600 rpm centrifugation protocol resulted in a slightly greater release of TGF-β1 (7,127 ng/mL) and PDGF (473,909 pg/mL). While the other samples resulted from 50,296 pg/mL (14 min with 1200 rpm) until 406.883 pg/mL (8 min with 800 rpm) of PDGF. The other results of TGF-β1 vary from 0,558 ng/mL (14 min with 1200 rpm) to 6,322 ng/mL (10 min with 600 rpm).
Conclusion: The highest concentration of PDGF and TGF-β1 were obtained from centrifugation process at 600 rpm for 8 minutes. Meanwhile, the lowest concentration of PDGF and TGF-β1 were obtained from centrifugation process at 14 min with 1200 rpm.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
References
Senzel L, Gnatenko DV, Bahou WF. The platelet proteome. Curr Opin Hematol. 2009;5:329–333.
Martinez-Zapata MJ, Marti-Carvajal AJ, Sola I, Exposito JA, Bolibar I, Rodriguez L, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;5.
Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28:429–39. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228.
Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-kit, Plateltex and one manual procedure. Vox Sang. 2009;97:110–118.
Le G, Kaux JF, Seidel L, Péters P, Gothot A, Albert A, Crielaard JM. Étude comparative de cinq techniques de préparation plaquettaire (platelet-rich plasma). Pathol Biol (Paris). 2011;59:157–160.
Raja VS, Naidu EM. Platelet-rich fibrin: evolution of a second-generation platelet concentrate. Indian J Dent Res. 2008;19(1):42.
Le AD, Enweze L, DeBaun MR, Dragoo JL. Platelet-rich plasma. Clin Sports Med. 2018;38:17-44.
Amable PR, Carias RBV, Teixeira MVT, da Cruz Pacheco Í, Corrêa do Amaral RJF, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:1-13.
Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–72.
Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg (Hong Kong). 2017;12.
Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc. 2017;25:485–92.
Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25:958–65.
Lana JFSD, Weglein A, Sampson SE, Vicente EF, Huber SC, Souza CV, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12:69–78.
Paterson KL, Nicholls M, Bennell KL, Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Musculoskelet Disord. 2016;17.
Reno C, Maietti E, Fantini MP, Savoia E, Manzoli L, Montalti M, et al. Enhancing COVID-19 vaccines acceptance: results from a survey on vaccine hesitancy in Northern Italy. Vaccines. 2021;9(4):378.
Pan MA, Lau TK, Tang Y, Wu YC, Liu T, Li K, et al. 16.7%-efficiency ternary blended organic photovoltaic cells with PCBM as the acceptor additive to increase the open-circuit voltage and phase purity. J Mater Chem A. 2019;7(36):20713–20722.
Robins RJ. Platelet rich plasma: Current indications and use in orthopaedic care. Med Res Arch.2017;5:1-17.
Fantini P, Jiménez R, Vilés K, Iborra A, Palhares MS, Catalán J, et al. Simple Tube Centrifugation Method for Platelet-Rich Plasma (PRP) Preparation in Catalonian Donkeys as a Treatment of Endometritis-Endometrosis. Animals. 2021;11(10):2918.
Zhan F, Zhu S, Liu H, Wang Q, Zhao G. Blood routine test is a good indicator for predicting premature rupture of membranes. J Clin Lab Anal. 2019;33(2):e22673.